binax now covid test drops|DIRECTIONS FOR RUNNING THE BINAXNOW COVID : manufacturer The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic . Lambertville, MI 48144. Get Directions. Phone: (734) 562-009.
{plog:ftitle_list}
Resultado da ESPN. Elche. 6° no 2ª Divisão Espanhola. Todos os resultados de Elche na temporada na ESPN (BR). Inclui todos os resultados de Elche nas .
The BinaxNOW COVID-19 Ag Card Home Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid .
The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic .
The BinaxNOW™ COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home .Since the launch of the BinaxNOW™ COVID-19 Antigen Self Test, Abbott has continued testing for product stability to extend the expiration date and have shared these results with the FDA. .The BinaxNOW TM COVID-19 Ag Card is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab .The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from direct .
SUMMARY and EXPLANATION of the TEST DIRECTIONS
BinaxNOW COVID-19 Ag Card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media. The .
The BinaxNOW™ COVID-19 Antigen Self Test, part number 195-160 or 195- 180, may now have a longer than labeled product expiry date. All BinaxNOW COVID-19 Antigen Self Test kits currently have a twenty-two-month expiry date.Purchase the BinaxNOW™ COVID‑19 Antigen Self Test at a retail store near you and perform the test with a simple nasal swab in the comfort and convenience of your home. Learn how to use the BinaxNOW™ COVID-19 Antigen Self Test.
The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from direct . These tests require precise steps—with BinaxNow, for instance, you must squeeze six drops of liquid into a small hole on the test card—and require visually interpreting the results.
DIRECTIONS FOR RUNNING THE BINAXNOW COVID
The BinaxNOW COVID-19 Antigen Self Test is a rapid lateral flow immunoassay for the qualitative detection of SARS-CoV-2 directly from anterior nasal swabs, without viral transport media. The BinaxNOW COVID-19 Antigen Self Test kit contains all components required to carry out an assay for SARS-CoV-2. PRINCIPLES of the PROCEDURE
The BinaxNOW™ COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home .The BinaxNow, BD Veritor, Flowflex, and Celltrion DiaTrust COVID-19 rapid antigen kits all contain this chemical. Sodium azide is a colorless, tasteless, and odorless powder that has been used as a propellant in automobile airbags, an . Our most popular test is our rapid antigen BinaxNOW COVID-19 Self Test which provides results in 15 minutes. WHAT IS ANTIGEN TESTING? Antigen tests detect proteins of the SARS-CoV-2 virus that form during the infection . The Walgreens by me had plenty of rapid tests, but other drugstores have sold out. Andrea Michelson A few weeks ago, I bought a BinaxNOW self-administered antigen test kit for .99.
BinaxNOW™ COVID
BinaxNOW™ COVID-19 Reagent Safety Data Sheet This document has been prepared in accordance with the SDS requirements of the OSHA Hazard Communication Standard 29 CFR 1910.1200 2020 08 17 EN (English US) 1/6 SECTION 1: Identification 1.1. Identification Product form : Mixture Product name : BinaxNOW™ COVID-19 Reagent 1.2. Don’t let the kit freeze.This can also damage the kit components. 2. Using straight from the fridge. The reagents (essential test kit ingredients) will not work properly at cold temperatures .
The BinaxNOW™ COVID-19 Antigen Self Test has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks could include theBinaxNOW ™ ANTIGEN SELF TEST COVID-19 support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and . drops slowly. 18. The Reagent .
The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at .
Open the smaller, top cap on the vial of liquid, then squeeze three drops of your sample into the collection area of the strip. Set a timer for 15 minutes. Don’t disturb the test strip during this time. . “When doing an at-home COVID test – or any test – on a child, it’s best to be honest with them,” he explained. “Be calm and .On Friday (March 18), the FDA issued an alert warning that at-home COVID-19 tests can cause harm if they are used improperly, for example, if the liquid test solution touches a person's skin or . High levels of covid-19 in the community also greatly reduce the chance any positive test you get is a false positive. MIT Technology Review encourages you to use rapid tests if you can find one.The BinaxNOW COVID-19 Self Test is identical to the professional-use test card, used since August 2020, and is the most studied and widely available rapid antigen test. With BinaxNOW authorized for over-the-counter frequent asymptomatic use, we are making testing directly available for fast results, when and where you need it.

test kits whose printed expiration dates have been extended. Customers may find that their test kits have printed lot . BinaxNOW™ COVID-19 Ag Self Test 15-month to 22-month shelf-life .To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab . Nasal Swabs (40): Sterile swabs for use with BinaxNOW COVID-19 Ag Card test Positive Control Swab (1): Non-infectious recombinant SARS-CoV-2 nucleocapsid antigen dried onto a swab If you get a negative result from a test beyond the expiration date, ensure the test doesn't have an extended expiration date. If it's beyond that expiration date, get another test or have a healthcare professional collect a .Abbott BinaxNOWTM COVID-19 Ag Card Test Helpful Testing Tips (continued) Before the Test . 1 . Store kit at 2-30°C (35.6-86° F). Do not freeze kits. Test kit reagents/cards must be at room temperature before use. If stored in a refrigerator, allow time to warm up to room temperature. Only the swabs provided with the kit are approved for use .
BinaxNOW: What You Need to Know
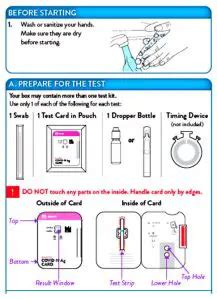
The BinaxNOW COVID-19 Antigen Self Test is a rapid lateral flow immunoassay for the qualitative detection of SARS-CoV-2 directly from anterior nasal swabs, without viral transport . an anterior nasal swab specimen is collected by the patient, then 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well .*Be sure there is a blue line present at the Control Line prior to running the test (discard the test card if there is no blue line). Step 1: Add the extraction reagent to the BinaxNOW COVID-19 Ag Card by holding the bottle vertically (NOT at an angle), hovering ½ inch above the TOP HOLE, slowly adding eight (8) drops.
The test can be used at home with a prescription through a virtually guided online service. And in April 2021, our BinaxNOW Self Test became available as an over-the-counter, self test at national retail stores. Each box contains two tests for frequent serial testing and has a suggested retail price of .99.
With reasonably good specificity and sensitivity, the speed and convenience of COVID-19 antigen tests have led to self-testing in schools, offices, and universities in the European Union (EU). Although self-testing can be beneficial and increase accessibility to testing, there are potential ways to confound a positive COVID-19 lateral flow test. JN.1, the now-dominant COVID-19 variant that accounts for nearly 86% of all currently circulating SARS-CoV-2 strains, may take longer to show a positive result on home antigen tests. Abbott’s BinaxNow COVID-19 Antigen Self Test kits come in boxes of two, four, or 10 tests. . Having received reports of people mistaking the small vials of test-kit solution for eye drops, the .
The BinaxNOW COVID -19 Ag Card 2 Home Test is intended for observed non-prescription self - use and/or, as applicable for an adult lay user testing another person aged 2 years or older in aThe BinaxNOW™ COVID-19 Ag Card provides COVID-19 test results quickly and easily. This simple, CLIA-waived test provides visual results in just 15 minutes. CONTACT. DIAGNOSTICS. ABOUT ABBOTT. . The BinaxNOW COVID-19 Ag Card test has received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA).* .
BinaxNOW ™ COVID
BinaxNOW COVID-19 Ag CARD HOME TEST KIT . Apply Fluid to Top Hole . A. Removedropper bottle cap. B. Hold dropper bottle straight over TOP HOLE, not at an angle. C. Put 6 DROPS into TOP HOLE. Do not touch card with tip. 6 drops . Note: False negative results may occur if less than 6 drops. of fluid is used.
BinaxNOW COVID
Resultado da Resumo do jogo Deportes Tolima vs. Alianza Petrolera Copa Colômbia, placar final 2-1, de 16 de agosto, 2023 em ESPN (BR).
binax now covid test drops|DIRECTIONS FOR RUNNING THE BINAXNOW COVID